Serial Vs Parallel Dilutions
A serial dilution is the stepwise of a in. Usually the at each step is constant, resulting in a of the in a fashion. A ten-fold serial dilution could be 1, 0.1 M, 0.01 M, 0.001 M. Serial dilutions are used to accurately create highly diluted solutions as well as solutions for resulting in with a. A tenfold dilution for each step is called a logarithmic dilution or log-dilution, a 3.16-fold (10 0.5-fold) dilution is called a half-logarithmic dilution or half-log dilution, and a 1.78-fold (10 0.25-fold) dilution is called a quarter-logarithmic dilution or quarter-log dilution.
Serial dilutions are widely used in experimental sciences, including,. Contents.In biology and medicine In and, besides the more conventional uses described above, serial dilution may also be used to reduce the concentration of microscopic organisms or cells in a sample. As, for instance, the number and size of that grow on an plate in a given time is concentration-dependent, and since many other diagnostic techniques involve physically counting the number of micro-organisms or cells on specials printed with grids (for comparing concentrations of two organisms or cell types in the sample) or wells of a given volume (for absolute concentrations), dilution can be useful for getting more manageable results. Serial dilution is also a cheaper and simpler method for preparing than. In homeopathy. Experiments in Microbiology, Plant Pathology and Biotechnology.
New Age Publishers, 2005, p. 69. Booth, C.; et al. Methods in microbiology 35.
Academic Press. P. 543. Weissmann, Gerald (2006). The FASEB Journal. 20 (11): 1755–1758. Retrieved 2008-02-01.
Slip Vs Ppp
Ernst, Edzard (November 2005). 'Is homeopathy a clinically valuable approach?' Trends in Pharmacological Sciences. 26 (11): 547–548.
Michael L. Bishop, Edward P. Fody, Larry E.
Clinical Chemistry: Principles, Procedures, Correlations. Lippincott Williams & Wilkins, 2004, p. 24.External links., Bates College.
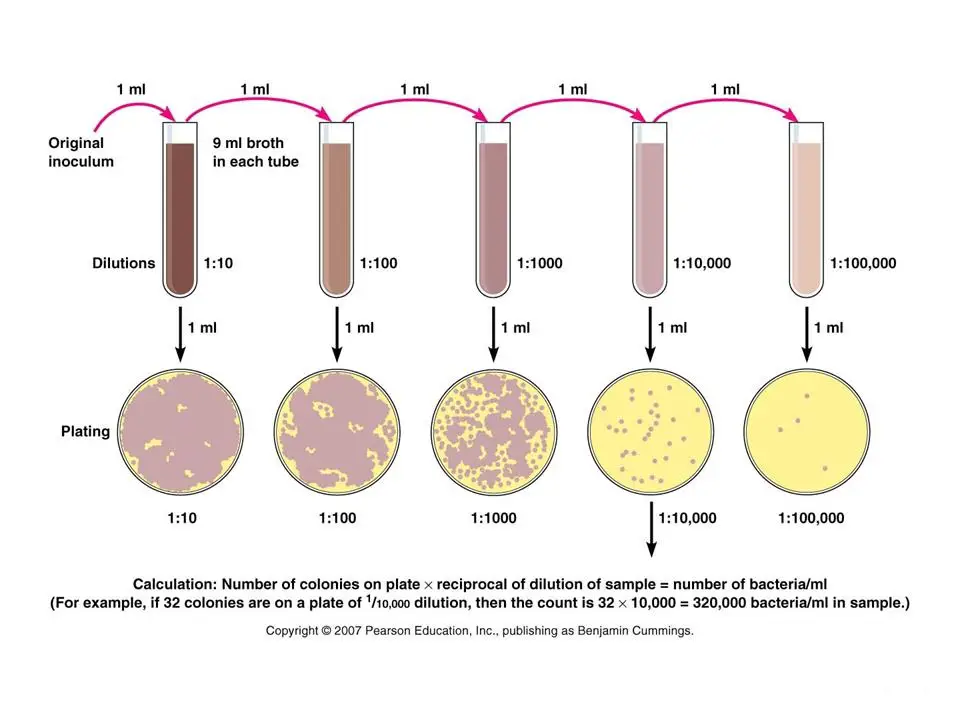
Each calibration standard solution is prepared based on the previous calibration standard. The process involves taking a portion of the previous standard and diluting it with the solvent to obtain the next calibration standard. The errors introduced with each successive dilution drops proportionately with the solution concentration. Preparing a series of calibration standards by this method reduces the amount of required time. Most calibration standards span a large range of concentrations, so the accuracy of the calibration standard prepared increases. Calibrations Solutions More Evenly Spaced.

Serial Vs Parallel Dilution

The dilution factor chosen for the series of calibration standards is achievable by using serial dilution. The progression of calibration standard concentration is always a geometric series.
Consider the example of making the first standard at 1/3 the concentration of the known, the next calibrant would be 1/9th the concentration of the known and the following two calibrants formed are 1/27th and 1/81st. This becomes a much greater advantage when the span of the calibration standards must cover several orders of magnitude in concentration.